Effects of ammonium hydroxide on the structure and gas adsorption of nanosized Zr-MOFs (UiO-66)
Abstract
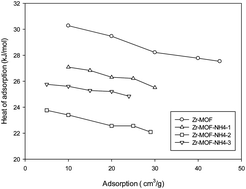
Several zirconium-based metal–organic frameworks (Zr-MOFs) have been synthesized using ammonium hydroxide as an additive in the synthesis process. Their physicochemical properties have been characterized by N2 adsorption/desorption, XRD, SEM, FTIR, and TGA, and their application in CO2 adsorption was evaluated. It was found that addition of ammonium hydroxide produced some effects on the structure and adsorption behavior of Zr-MOFs. The pore size and pore volume of Zr-MOFs were enhanced with the additive, however, specific surface area of Zr-MOFs was reduced. Using an ammonium hydroxide additive, the crystal size of Zr-MOF was reduced with increasing amount of the additive. All the samples presented strong thermal stability. Adsorption tests showed that capacity of CO2 adsorption on the Zr-MOFs under standard conditions was reduced due to decreased micropore fractions. However, modified Zr-MOFs had significantly lower adsorption heat. The adsorption capacity of carbon dioxide was increased at high pressure, reaching 8.63 mmol g−1 at 987 kPa for Zr-MOF-NH4-2.

 Please wait while we load your content...
Please wait while we load your content...