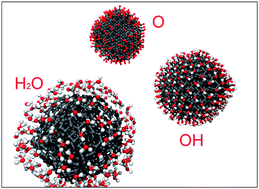
Understanding nanodiamond functionalisation is of great importance for biological and medical applications. Here we examine the stabilities of oxygen, hydroxyl, and water functionalisation of the nanodiamonds using the self-consistent charge density functional tight-binding simulations. We find that the oxygen and hydroxyl termination are thermodynamically favourable and form strong C–O covalent bonds on the nanodiamond surface in an O2 and H2 gas reservoir, which confirms previous experiments. Yet, the thermodynamic stabilities of oxygen and hydroxyl functionalisation decrease dramatically in a water vapour reservoir. In contrast, H2O molecules are found to be physically adsorbed on the nanodiamond surface, and forced chemical adsorption results in decomposition of H2O. Moreover, the functionalisation efficiency is found to be facet dependent. The oxygen functionalisation prefers the {100} facets as opposed to alternative facets in an O2 and H2 gas reservoir. The hydroxyl functionalisation favors the {111} surfaces in an O2 and H2 reservoir and the {100} facets in a water vapour reservoir, respectively. This facet selectivity is found to be largely dependent upon the environmental temperature, chemical reservoir, and morphology of the nanodiamonds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...