The colloidal stability of fluorescent calcium phosphosilicatenanoparticles: the effects of evaporation and redispersion on particle size distribution
Abstract
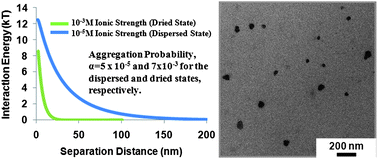
Understanding the colloidal stability of
- This article is part of the themed collection: Optical Materials

 Please wait while we load your content...
Please wait while we load your content...