Mesoscale crystallization of calcium phosphatenanostructures in protein (casein) micelles†
Abstract
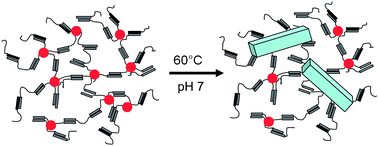
Aqueous micelles of the multi-
- This article is part of the themed collection: Crystallization and Formation Mechanisms of Nanostructures

 Please wait while we load your content...
Please wait while we load your content...