Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: synthesis and medicinal significance
Abstract
Covering: 1962 to 2014
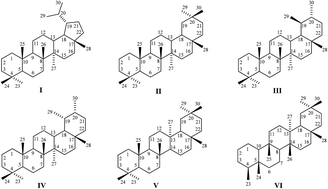
Triterpenoids are natural compounds with a variety of biological activities and are usually produced by plants as secondary metabolites. In recent decades, scientists focused on the properties of triterpenoids have discovered many activities, such as antitumor, antiviral, antimicrobial, anti-inflammatory and others. Thousands of new triterpenoids with various skeletal modifications have now been synthesized. One of the most important modifications is the formation of a new heterocyclic ring. The simple fact that the vast majority of currently used drugs are heterocyclic compounds has encouraged a lot of researches to synthesize analogous triterpenoid derivatives in order to find new molecules with higher activities and consequently optimize their pharmacological profile. The biological properties of triterpenoid heterocycles are very promising and many of them have been studied, especially as antitumor agents. This review is mainly focused on the synthesis of various types of nitrogen and occasionally sulfur-containing heterocyclic triterpenoids for their potential use as future drugs in medicine and in addition discusses their overall biological activities.

 Please wait while we load your content...
Please wait while we load your content...