Properties of an unusual heme cofactor in PLP-dependent cystathionine β-synthase†
Abstract
Covering: up to 2006
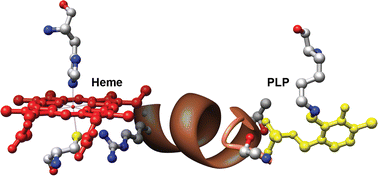
Cystathionine β-synthase is a pyridoxal phosphate (PLP)-dependent hemeprotein, which catalyzes the condensation of serine and homocysteine to give cystathionine, the first step in the transsulfuration pathway. The association of heme with this enzyme in some organisms, including humans, is novel since this combination of cofactors has not been seen in any other protein to date. While the role of PLP in the reaction is suggested by the beta replacement reaction that is catalyzed, the role of the heme is not yet well established. Spectroscopic studies reveal that the heme iron is coordinately saturated in the ferric and ferrous states and is relatively inert to ligand exchange in the oxidized state. The in vitro activity of cystathionine β-synthase is sensitive to the redox state of the heme and is higher in the ferric form. Both CO and NO bind to ferrous heme and inhibit the enzyme. The crystal structure of the protein reveals that the heme is ∼20 Å away from the active site and suggests a pathway for signal transduction between the heme and active sites. In this review, the spectroscopic and kinetic properties of the heme are discussed, which supports a regulatory role for this unexpected organometallic cofactor in a PLP-dependent enzyme.
- This article is part of the themed collection: Heme

 Please wait while we load your content...
Please wait while we load your content...