Modulating the DNA cleavage ability of copper(ii) Schiff bases through ternary complex formation†
Abstract
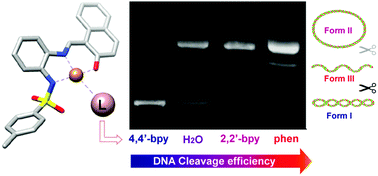
Copper(II) complexes with the potentially tridentate Schiff bases N-[(3-ethoxy-2-hydroxyphenyl)methylidene]-N′-tosylbenzene-1,2-diamine (H2L1) and N-[(2-hydroxynaphthanyl)methylidene]-N′-tosylbenzene-1,2-diamine (H2L2) have been synthesized by electrochemical oxidation of the metal in an electrochemical cell containing a solution of the corresponding ligand in acetonitrile. Adducts of these compounds with 2,2′-bipyridine (2,2′-bpy), 4,4′-bipyridine (4,4′-bpy) or 1,10-phenanthroline (phen) were also obtained. The complexes have been characterized by microanalysis, mass spectrometry, EPR, IR and UV-Vis spectroscopies, as well as DFT calculations. The ligand H2L1 and the compounds [CuL1(H2O)]·H2O (1), [Cu2L12(4,4′-bpy)] (3), [CuL1(phen)] (4), [CuL2(2,2′-bpy)] (6), and [Cu2L22(4,4′-bpy)3] (7) have also been characterized by X-ray diffraction. The copper compounds 1, 4 and 6 are mononuclear with a square planar geometry around the metal for 1 and square pyramidal for 4 and 6. However, 3 and 7 are dinuclear complexes with a 4,4′-bpy ligand bridging two copper atoms with square planar (3) or square pyramidal (7) coordination. The supramolecular structures were studied by means of Hirshfeld surface analysis. The adducts of phen and 2,2′-bpy exhibit enhanced DNA cleavage ability compared to the [CuL(H2O)] complexes (L = L1 or L2), while the binuclear entities formed by 4,4′-bpy coordination present reduced nuclease efficiency.


 Please wait while we load your content...
Please wait while we load your content...