High strength and anti-fatigue nanocomposite hydrogels prepared via self-initiated free radical polymerization triggered by daylight
Abstract
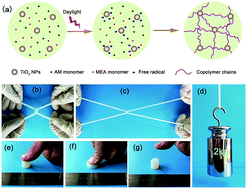
Nanocomposite hydrogels (NC gels) consisting of an acrylamide (AM) and 2-methoxyethyl acrylate (MEA) copolymer and titania nanoparticles (TiO2 NPs) as both inorganic cross-linking agents and photo-initiators were synthesized. Especially, the formation of hydrogels can be realized under daylight without using any initiating systems or special treatments. The resultant NC gels displayed outstanding mechanical performance including high extensibility (elongation at break >1000%), high strength (fracture stress up to 630 kPa), high toughness (∼4.64 MJ m−3), and anti-fatigue properties due to hydrogen bonding interactions between TiO2 NPs and polymer chains. Furthermore, the hydrogels also revealed considerable mechanical strength in the swollen state because of their relatively low swelling ratios. It is hoped that this novel type of NC gels could promote current hydrogel research and broaden their practical applications.


 Please wait while we load your content...
Please wait while we load your content...