Novel antitumor copper(ii) complexes designed to act through synergistic mechanisms of action, due to the presence of an NMDA receptor ligand and copper in the same chemical entity
Abstract
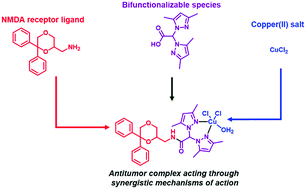
In the present article the 1,4-dioxane derivative 1, a potent noncompetitive NMDA receptor antagonist, showed cytotoxic activity in the MCF7 breast cancer cell line significantly higher than those of the functionally related compounds (S)-(+)-ketamine and MK-801. Encouraged by this result and considering that copper complexes have been highlighted to be promising anticancer agents, the NMDA receptor ligand 1 was linked to the bifunctionalizable species 2 and 3, affording the conjugated derivatives 4 and 5 that were used for the preparation of the stable Cu(II) complexes 6 and 7. All the compounds were evaluated against a panel of human cancer cell lines derived from solid tumors. Complex 7 showed the best antitumor activity in all the studied cell lines. This result suggests that 7 might act through synergistic mechanisms of action due to the presence of the NMDA ligand 1 and copper(II) in the same chemical entity. Furthermore, the cellular mechanisms affected by complex 7 were assessed through cytofluorimetric and western blot analyses. Data suggested the induction of cell death through paraptosis mediated by endoplasmic reticulum (ER) stress.


 Please wait while we load your content...
Please wait while we load your content...