Density functional theory modelling of protective agents for carbonate stones: a case study of oxalate and oxamate inorganic salts†
Abstract
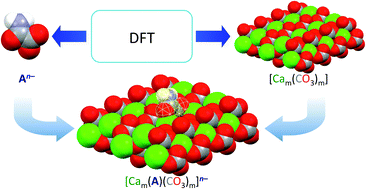
Sulphur and nitrogen oxide pollutants cause acid rain that can eventually lead to the dissolution of calcite in marble and limestones. Calcium oxalate is an inorganic protective agent, which is obtained by treatment with ammonium oxalate. The functionalization of oxalic acid to give monoesters and monoamides (oxamates) allows tailoring the solubility of the relevant ammonium and calcium salts. In this context, theoretical calculations carried out at the Density Functional Theory (DFT) level were exploited to investigate the capability of oxalate, methyloxalate, phenyloxalate, oxamate, methyloxamate, and phenyloxamate to interact with the calcium carbonate lattice. An in-depth validation based on the structural data showed that DFT calculations with the PBE0 functional along with a single or triple-zeta def2 basis set allow understanding the different reactivity of the oxalate and oxamate derivatives and their efficiency in interacting with stones containing calcium carbonate, such as Carrara marble and biomicritic limestones.


 Please wait while we load your content...
Please wait while we load your content...