Functional group effect of isoreticular metal–organic frameworks on heavy metal ion adsorption†
Abstract
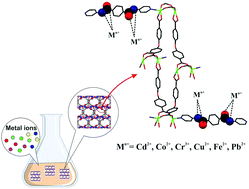
Evaluating the relationship between structure and function of metal–organic frameworks (MOFs) is an interesting issue that is discussed in this work. Here, four isoreticular 3D porous zinc(II) MOFs with pcu topology, including [Zn(oba)(4-bpmb)0.5]·(DMF)1.5 (TMU-6), [Zn(oba)(bpmn)0.5]·(DMF)1.5 (TMU-21), [Zn2(oba)2(bpfb)]·(DMF)5 (TMU-23) and [Zn2(oba)2(bpfn)]·(DMF)2 (TMU-24), H2oba = 4,4′-oxybisbenzoic acid, bpmb = N,N′-bis-(4-pyridylmethylene)-1,4-benzenediamine, bpmn = N,N′-bis-(4-pyridylmethylene)-1,5-naphthalenediamine, bpfb = N,N′-bis-(4-pyridylformamide)-1,4-benzenediamine and bpfn = N,N′-bis(4-pyridylformamide)-1,5-naphthalenediamine, containing imine- (TMU-6 and TMU-21) and amide- (TMU-23 and TMU-24) decorated pores, were successfully synthesized by a mechanochemical method. Then, adsorption efficiency of these four MOFs for some heavy metal ions was studied to evaluate effects of the types of functional groups of the pillars in different MOFs on the adsorption process. The results indicated that amide-decorated MOFs show a better adsorption efficiency toward metal ions than the imine-decorated MOFs. In the following, TMU-23 was used as an efficient sorbent for extraction and removal of some heavy metal ions (Co2+, Cd2+, Cu2+, Cr3+, Fe2+, and Pb2+) and its analytical performance was evaluated and determined. In addition, DFT calculations were performed on possible coordination modes between cations and simplified functional groups of the related pillars in each MOF and a probable interaction mechanism of the MOFs and the metal ions was evaluated.


 Please wait while we load your content...
Please wait while we load your content...