Iodine promoted iodosulfonylation of alkynes with sulfonyl hydrazides in an aqueous medium: highly stereoselective synthesis of (E)-β-iodo vinylsulfones†
Abstract
A catalyst-free and metal-free sulfonylation reaction for the preparation of (E)-β-iodo vinylsulfones from alkynes with sulfonyl hydrazides and molecular iodine was efficiently developed under mild and environmentally benign conditions, in water without any ligand or catalyst. The reaction gave a range of structurally diverse β-iodo vinylsulfones with excellent E selectivities and high yields.
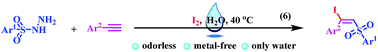


 Please wait while we load your content...
Please wait while we load your content...