Triphenylstibine-substituted Fischer carbene complexes of tungsten(0): synthesis, structure, DFT and electrochemistry†
Abstract
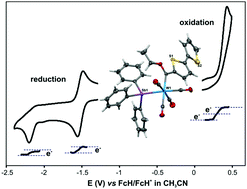
The synthesis, solid-state structure, and electrochemical behaviour of triphenylstibine-containing Fischer carbene complexes of tungsten(0) of the type [(SbPh3)(CO)4WC(OEt)(Ar)] with Ar = 2-thienyl (1), 2-furyl (2), 2-(N-methyl)pyrrolyl (3) or 2,2′-bithienyl (4) are reported. A density functional theory study of all the possible conformations and isomers of the various moieties in these complexes show that while the 2-furyl group adopts an anti-orientation relative to the ethoxy group, the thienyl and 2-(N-methyl)pyrrolyl adopt a cis-orientation. The preferred orientation of the aryl groups was confirmed by the solid-state structures. An electrochemical study of the complexes reveals that the decreasing order of the metal-centred oxidation and the carbene–carbon reduction of the [(SbPh3)(CO)4WC(OEt)(Ar)] complexes is: Ar = C4H3S > C4H3O > C4H3NMe. The electrochemical study further reveals substitution of a CO group in [(CO)5WC(OEt)(Ar)] with SbPh3, leads to a lowering of the metal-centred oxidation and the carbene–carbon reduction potential of ca. 0.27 and 0.13 V, respectively.


 Please wait while we load your content...
Please wait while we load your content...