Two luminescent transition-metal–organic frameworks with a predesigned ligand as highly sensitive and selective iron(iii) sensors†
Abstract
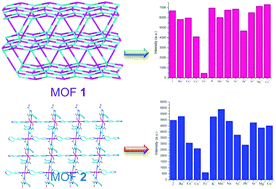
To obtain new structural MOFs with the desired properties, a multifunctional organic ligand, 2-(4-cyanophenyl)-1H-imidazole-4,5-dicarboxylic acid (p-CNPhH3IDC) has been designed and prepared. The optimized geometry and natural bond orbital (NBO) charge distributions of the free p-CNPhH3IDC ligand have been calculated by the theoretical method. Its strong coordination ability was confirmed by experimental results. In addition, two new metal–organic frameworks [Cd(p-CNPhHIDC)(4,4′-bipy)0.5]n (1) and [Zn(p-CNPhHIDC)(4,4′-bipy)]n (2) (4,4′-bipy = 4,4′-bipyridine), have been hydro(solvo)thermally synthesized and structurally characterized by elemental analysis, infrared spectroscopy, powder X-ray diffraction and single crystal X-ray diffraction. Their chemical-stable, thermal and solid-state photoluminescent properties have been investigated. Luminescence studies reveal that both the complexes not only can highly selectively sense the Fe3+ ion through fluorescence quenching but also resist interference from other metals. More importantly, the response time is very fast. This suggests that the possible sensing mechanism is the competitive absorption of excitation energy.


 Please wait while we load your content...
Please wait while we load your content...