Sigma-hole interactions in the molecular and crystal structures of N-boryl benzo-2,1,3-selenadiazoles†
Abstract
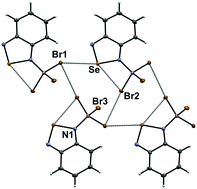
N-Bonded adducts of benzo-2,1,3-selenadiazole with BX3 (X = Ph, F, Cl, Br) were prepared and crystallographically characterized. The structures of the 1 : 1 adducts of BF3 and BCl3 demonstrate dimerization through the [Se–N]2 supramolecular synthon and enhancement of the corresponding Se⋯N chalcogen bonding interactions. However, the structures of the BPh3 and BBr3 compounds indicate that other supramolecular interactions can efficiently compete and inhibit [Se–N]2 dimerization. In the case of the BPh3 adduct, dispersion favors a dimer featuring Se⋯C interactions. In the crystal of the BBr3 derivative, the cooperative effect of Se⋯N chalcogen bonding and Br⋯Br halogen bonding interactions lead to a network structure. A 1 : 2 adduct could be isolated only in the case of BCl3; its structure features short intramolecular Se⋯Cl interactions resulting from the enhanced electrophilic character of the chalcogen atom.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...