Synthesis of petal-like δ-MnO2 and its catalytic ozonation performance†
Abstract
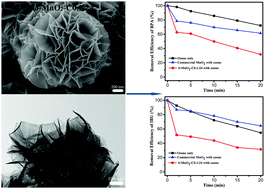
Petal-like δ-MnO2 microspheres were successfully synthesized by a very simple hydrothermal method with potassium permanganate (KMnO4) as the only raw material. By adjusting the initial concentration of KMnO4 solution and the time of the hydrothermal reaction, the morphology and crystallinity of δ-MnO2 can be controlled. The synthesized δ-MnO2 microspheres were characterized by XRD, SEM, TEM, TG/DSC, BET, ICP-AES and XPS. The catalytic ozonation performance of δ-MnO2 prepared by a hydrothermal reaction at 160 °C for 24 h using 0.1 mol L−1 KMnO4 solution was tested. Its catalytic activity is excellent. The degradation efficiencies of bisphenol A and ibuprofen using 0.1 g L−1 δ-MnO2 as the catalyst were 68.2% and 68.5% in 20 min reaction time. The results were much better than those of individual ozone treatment and with commercial MnO2 as the catalyst. The formation of hydroxyl radicals was not the main reason for the rapid degradation of organic compounds in the catalytic reaction with δ-MnO2 as the catalyst. The strong interaction among the catalyst surface, ozone and organic molecules was the important reason for catalysis based on the related experimental results.


 Please wait while we load your content...
Please wait while we load your content...