2,2′-Bipyridine and hydrazide containing peptides for cyclization and complex quaternary structural control†
Abstract
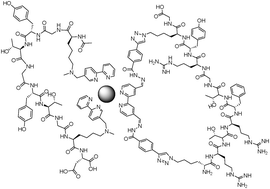
A synthetic peptide containing two Nε-methyl lysines (Ac-K(Nε-Me)GYTGYTGK(Nε-Me)D-OH) was alkylated with bipyridine (bipy) ligands substituted at the fifth (MP-5) and sixth (MP-6) positions, thereby creating Ac-K(Nε-Me, Nε-Bipy)GYTGYTGK(Nε-Me, Nε-Bipy)D-OH. Peptides with a bipyridine at the 6-position did not bind to Fe2+ and Zn2+. Peptides with a bipyridine at the 5-position bound these metals, and in the presence of one equivalent of a free bipy derivative folded into a macrocycle. Further, the free bipy derivative could also contain a cyclized peptide derived from hydrazone formation, resulting in a complex but controlled quaternary peptide structure.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...
