Novel composite films of polysaccharides and glutathione capped zinc selenide (GSH@ZnSe) quantum dots for detection of Cd2+ and Cu2+†
Abstract
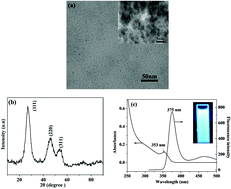
Water-soluble glutathione capped zinc selenide (GSH@ZnSe) quantum dots (QDs) were employed to develop composite films with two polysaccharides, i.e. positively charged chitosan (CS) and negatively charged xanthan gum (XG), respectively. Both fluorescent and electrochemical strategies were used to detect Cd2+ and Cu2+. The composite films were deposited on a glass substrate and the detection of Cd2+ and Cu2+ was achieved by monitoring fluorescence quenching of the QDs. Compared to XG, which was highly soluble in water, CS acted as an insoluble matrix for QDs and provided good adhesion to the glass substrate. The fluorescence of GSH@ZnSe/CS decreased linearly with the increase in concentration of metal ions, suggesting that the material could be a potential candidate for quantitative detection of Cd2+ and Cu2+. Compared with QD solutions, such a composite film could prominently facilitate the exploitation of QD sensors for device construction. The composites were also deposited onto MWCNT modified glassy carbon (GC) electrodes to detect the electrochemical oxidation of Cd2+ and Cu2+. Electrodes with GSH@ZnSe/XG/MWCNT film had good stability and demonstrated a sensitive detection of Cd2+ and Cu2+ with stripping peak intensity increasing linearly with metal ion concentration. In addition, Cd2+ and Cu2+ could be resolved in a single linear sweep voltammogram (LSV), which was impossible when using the fluorescence quenching technique.


 Please wait while we load your content...
Please wait while we load your content...