Supercritical CO2 assisted synthesis of sulfur-modified zeolites as high-efficiency adsorbents for Hg2+ removal from water†
Abstract
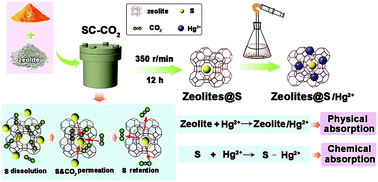
A novel synthetic strategy has been successfully developed, which utilizes supercritical CO2 (SC-CO2) to fabricate sulfur-modified zeolites (zeolites@S) for Hg2+ removal from water. Owing to the low viscosity, high diffusivity and excellent dissolving capacity of SC-CO2 as well as the nontoxicity, noninflammability and being a low-cost solvent it exhibits the strong ability to expand the crystal structure of zeolite and the high-efficiency transfer of sulfur in zeolite matrices. Benefiting from this unique microstructure, zeolites@S adsorbents provide abundant pores and voids for absorbing Hg2+, and synchronously offer extra active sites for chemically trapping Hg2+ with sulfur. The pseudo-first-order kinetic/pseudo-second-order kinetic models and Langmuir/Freundlich isotherm models have been employed to study the adsorption behaviors and adsorption kinetic parameters. The results demonstrated that zeolites@S-15 (sulfur content = 15 wt%) exhibited the highest adsorption capacity and the best kinetic parameters for Hg2+ relative to other samples. This work will not only provide important reference for the rational design of zeolite based adsorbents for Hg2+ removal from aqueous solution, but also pave a new way to boost the commercialization of low-cost and high-efficiency functionalized adsorbents for purifying wastewater.


 Please wait while we load your content...
Please wait while we load your content...