Structural preferences in strong anion–π and halogen-bonded complexes: π- and σ-holes vs. frontier orbitals interaction†
Abstract
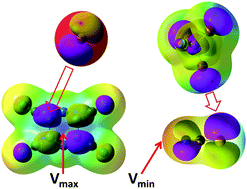
X-ray crystallographic and computational analyses of supramolecular associates between the tetracyanopyrazine π-acceptor and halide anions, as well as between brominated electrophiles and pseudohalides, show consistent deviations of the donor/acceptor contacts from the areas of most positive or negative potentials on the surfaces of the interacting species. The geometries of all these anion–π and halogen-bonded complexes suggest bonding overlap of the frontier orbitals of the reactants. In accordance with the experimental data, TD-DFT computations produced intense absorption bands in the UV-Vis spectra of the fully-optimized complexes. Also, their NBO analysis indicated substantial donor/acceptor charge transfer. In contrast, the intensities of the absorption bands and amount of charge transfer calculated for the hypothetical associates in which donor/acceptor contacts occurs at the most positive and negative surface potentials were much lower (if any). Overall, the anion–π and halogen-bonded complexes under study represent an example of supramolecular associates in which electrostatic and molecular-orbital models predict different donor/acceptor arrangements. Their analysis suggests a vital role of orbital interactions in a subtle balance of the intermolecular forces that determine the structural features of these complexes.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...