Mg1−xCoxLi2(3,5-pdcH)2(DMF)2 (x = 0, 0.285, 0.575, 1): a series of heterometallic coordination polymers doped with magnetic Co2+ ions†
Abstract
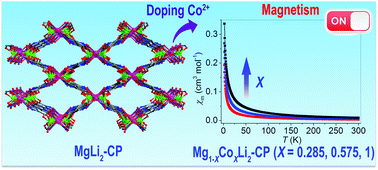
The introduction of heterometal ions into coordination polymers (CPs) could yield novel structural types and thus unprecedented properties. This work reports on the syntheses, characterizations and magnetic properties of a series of heterometallic CPs (HCPs), namely Mg1−xCoxLi2(3,5-pdcH)2(DMF)2 (x = 0 (1), 0.285 (2), 0.575 (3) and 1 (4); 3,5-pdcH3 = pyrazole-3,5-dicarboxylic). The CPs are isostructural to each other with a three dimensional neutral framework of apo type topology, which is constructed by 3,5-pdcH2− ligands interconnecting infinite chain-like SBUs of [MLi2(COO)4(DMF)2]n (M = Mg (1), Mg0.715Co0.285 (2), Mg0.425Co0.575 (3) and Co (4)). Compound 1 represents a rare example of an s–s block HCP based on lightweight Mg2+ and Li+ ions. Compound 1 is magnetically inactive, while the successful doping of Co2+ ions into Mg2+ sites endows the resulting heterometallic compounds with antiferromagnetic behaviour. The magnetic behaviour of compounds 2–4 was analyzed and was shown to come from the zero field split or the spin–orbit coupling of the high-spin mononuclear Co2+ ions. Compounds 2 and 3 represent a rare type of HCP which simultaneously contains independent metal ions (Li+) and co-occupied ones (Mg2+ and Co2+) in different crystallographic sites.


 Please wait while we load your content...
Please wait while we load your content...