Synthesis, crystal structures, one/two-photon optical properties and bioimaging application of two organic molecules with D–A and D–π–A models containing 6-phenyl-2,2′-bipyridine†
Abstract
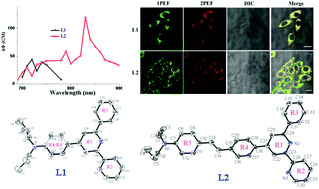
Two novel organic compounds with D–A (L1) and D–π–A (L2) models containing 4-(N,N-dipropylamino)phenyl [donor group (D)] and 6-phenyl-2,2′-bipyridine [acceptor (A)] groups were synthesized and characterized by IR, 1H NMR, 13C NMR, MS and single crystal X-ray diffraction. Their photophysical properties were systematically investigated using both experiments and theoretic calculations. It was found that the two-photon action cross-section (δΦ) of the D–π–A model molecule (δΦL2 = 119 GM) was approximately three times larger than that of the D–A type molecule (δΦL1 = 43 GM). Furthermore, their cytotoxicity and applications in bioimaging were determined. The results showed that L1 and L2 could be used as cytoplasmic dyes with high stability and low cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...