Reaction-based bi-signaling chemodosimeter probe for selective detection of hydrogen sulfide and cellular studies†
Abstract
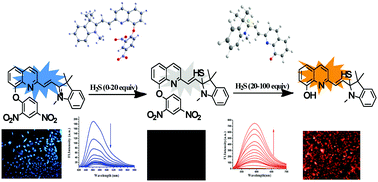
A new quinoline-indolium-based chemical probe (DPQI) was synthesized and characterized for selective detection of hydrogen sulphide (H2S). Probe DPQI displays highly selective and sensitive detection of hydrogen sulphide over other allied anions and thiol-containing amino acids in aqueous-DMSO at physiological pH. The probe DPQI acts as a bi-signaling fluorescent chemodosimeter for selective detection of hydrogen sulphide when excited at different wavelengths. The selectivity was guaranteed by the use of the unique nucleophilicity of HS− for nucleophilic addition to the most electrophilic positively charged centre followed by thiolysis of the dinitrophenyl ether to yield DNB-SH and a fluorescent phenoxide moiety, which led to the fluorescence emission ‘turn on’ and characteristic visual fluorescent color change behavior of the sensing system. This is why the probe DPQI showed different optical properties at varying concentrations of hydrogen sulphide. The structural and electronic properties of the probe (DPQI) and its thiolysis product have been demonstrated using ab initio density functional theory (DFT) combined with time-dependent density functional theory (TDDFT) calculations. Utilizing this probe, we have successfully detected hydrogen sulphide in live cells.


 Please wait while we load your content...
Please wait while we load your content...