Heterocyclic amino acids as fluorescent reporters for transition metals: synthesis and evaluation of novel furyl-benzoxazol-5-yl-l-alanines
Abstract
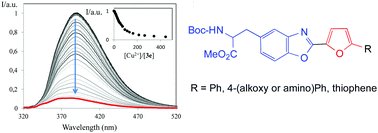
Unnatural alanine derivatives bearing a benzoxazole moiety at the side chain, acting simultaneously as the coordinating and reporting unit, via fluorescence changes, were synthesised with a furan ring at position 2, substituted with different (hetero)aryl substituents, in order to tune the photophysical and chemosensory properties of the resulting compounds. The amino acid derivatives were prepared in protected form at their N- and C-termini with tert-butoxycarbonyl urethane and methyl ester protecting groups, respectively. The novel furyl-benzoxazol-5-yl-L-alanines were evaluated for the first time in the recognition of several transition metal cations with environmental, medicinal and analytical interest such as Co2+, Cu2+, Zn2+ and Ni2+, in acetonitrile solution. The spectrofluorimetric titrations showed that the novel benzoxazolyl-alanines 3a–g were sensitive, although not selective, fluorimetric chemosensors for the above mentioned cations, with the best result being obtained for N,N-diethylamino substituted alanine 3d in the sensing of Cu2+. The heterocyclic system at the side chain was identified as the key factor in the recognition event of the metal cations, through its electron donor atoms. The encouraging photophysical and metal ion sensing properties of these furyl-benzoxazolyl-alanines suggest that they can be used to obtain bioinspired fluorescent reporters for metal ions such as peptides/proteins with chemosensory/probing abilities.


 Please wait while we load your content...
Please wait while we load your content...