Different self-assemblies and absorption–emission properties of the picrate salts of aromatic amine or heterocycle linked oximes†
Abstract
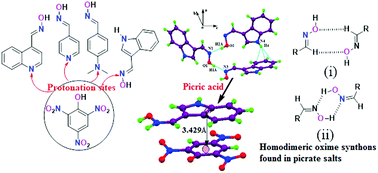
The supramolecular oxime synthons in the self-assemblies of picrate salts of 4-(N,N-dimethylaminophenyl)aldoxime, quinoline-4-carbaldoxime and pyridine-4-carbaldoxime are different. In these salts, protonation in each case occurs at a nitrogen atom located at a remote site of the oxime. These results suggest that synthon analysis provides descriptors rather than predicting self-assemblies. The self-assembly of the picrate salt of 4-(N,N-dimethylaminophenyl)aldoxime lacks an oxime homodimer. The R22(8) type homodimeric sub-assemblies of oximes are observed in the picrate salt of quinoline oxime, whereas a similar picrate salt of pyridine-oxime has R22(4) type oxime homodimers. Water molecules in this salt act as filler molecules for a tight-packed structure and have a role in deciding the nature of oxime synthons. Protonation at a nitrogen atom of the oxime was observed in the picrate salt of indole-3-carbaldoxime. This salt undergoes charge-transfer between the aromatic rings. The differential scanning calorimetry data establish the higher melting points of salts over the parent components and exothermic decompositions just above or near the respective melting temperature. The charge-transfer interactions of the picrate salt of indole-3-carbaldoxime cause a color difference from other salts. The emission spectrum of each salt is invariably quenched in solution or in the solid state. The relative ability of different nitro-aromatic compounds to quench emissions of the oxime derivatives enables them to be differentiated in solution. It is shown that the modification of the ground state by charge-transfer interaction is not the sole factor to cause fluorescence quenching in these salts, but excited state proton transfer plays a decisive role.


 Please wait while we load your content...
Please wait while we load your content...