Novel mono, di and tri-fatty acid esters bearing secondary amino acid ester head groups as transdermal permeation enhancers†
Abstract
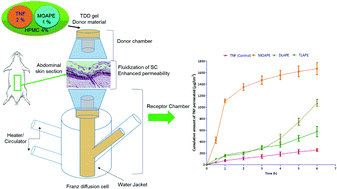
The use of chemical permeation enhancers (CPEs) has widened the pool of drugs that can be delivered via the transdermal route. This study explored the synthesis and characterization of novel mono, di and tri-fatty acid (FA) esters bearing β-alanine t-butyl ester head groups as transdermal permeation enhancers using tenofovir (TNF) as a model drug. The synthesized compounds were non-toxic to mammalian cells confirming their safety for pharmaceutical applications. All the synthesized derivatives displayed better transdermal permeation enhancement capabilities as compared to their respective individual FAs. The results showed that there was no correlation between the number of aliphatic carbon chains and the enhancement ratio (ER). The mono oleate derivative (MOAPE (4II)) displayed the greatest ER for TNF (5.87) at 1% w/w. Histological investigations of the rat skin treated with MOAPE (4II) revealed fluidization of the stratum corneum. Histological and transepithelial electrical resistance (TEER) studies corroborated with the findings of the in vitro permeation experiments and revealed that there was no significant change in the viable epidermis of the skin after 1% MOAPE (4II) exposure. The TEER findings also suggested that the permeation enhancement effects of MOAPE (4II) were not permanent and the results indicated a return towards original skin integrity after removal of the enhancer formulation.


 Please wait while we load your content...
Please wait while we load your content...