Influence of Na and Nb co-substitution on electrochemical performance of ternary cathode materials for Li-ion batteries
Abstract
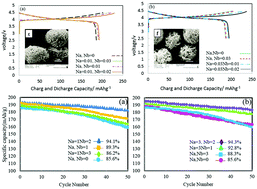
In the present study, Li1−xNax(Ni0.6Co0.2Mn0.2−yNby)O2, a novel layered cathodic compound, is synthesized using an economically feasible polymer pyrolysis method. Microstructure, morphology and electrochemical properties of the synthesized compounds are studied by XRD, SEM, charge–discharge tests, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The results reveal that substitution of Nb and Na can improve the cycling properties. The replacement of Nb can play an important role in improving maintenance capacity and cycling behavior. In addition, the Li-ion diffusability is significantly improved as a result of Na doping, which can be related to the reduction in Li+/Ni2+ cation mixing and its ionic radius. The highest retention percentage of 94.3% and discharge capacity were achieved for the sample containing Na = 0.03 and Nb = 0.02.


 Please wait while we load your content...
Please wait while we load your content...