Effects of appended hydroxyl groups and ligand chain length on copper coordination and oxidation activity†
Abstract
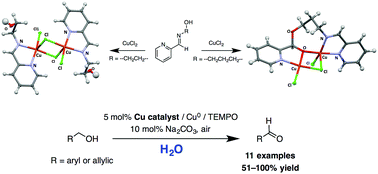
Treatment of a series of (imino)pyridine ligands bearing appended hydroxyl groups 2-((pyridin-2-ylmethylene)amino)phenol (Hpyph), 2-((pyridin-2-ylmethylene)amino)ethanol (Hpyet), and 3-((pyridin-2-ylmethylene)amino)propanol (Hpypr) with one equiv. of CuCl2·2H2O afforded the corresponding Cu(II) complexes in low to moderate yields. The crystal structure of (μ-Cl)2[CuCl(κ2-N,N-Hpyet)]2 reveals a symmetric dinuclear structure with the bidentate N,N-coordination mode of (imino)pyridine with no Cu–OH interaction. On the other hand, the dinuclear Cu(II) complex of the related propyl ligand Hpypr possesses a significantly different crystal structure involving nucleophilic addition of the hydroxyl group to the aldehyde group of 2-pyridinecarboxaldehyde. The Cu complex/Cu0/TEMPO/Na2CO3 (TEMPO = 2,2,6,6-tetramethylpiperidinyl-1-oxyl) catalyst system generally exhibited good activity for aerobic oxidation of benzyl alcohol to benzaldehyde in H2O at room temperature. The dinuclear Cu(II) complex (μ-Cl)2[CuCl(κ2-N,N-Hpyet)]2 was demonstrated as an effective catalyst toward aerobic oxidation of various benzyl alcohol derivatives, cinnamyl alcohol, and 2-thiophenemethanol.


 Please wait while we load your content...
Please wait while we load your content...