Enantioselective synthesis of substituted α-aminophosphonates catalysed by d-glucose-based crown ethers: pursuit of the origin of stereoselectivity†
Abstract
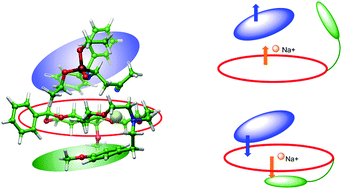
Several monoaza-15-crown-5 type macrocycles annelated to phenyl-β-D-glucopyranoside were applied as chiral catalysts in enantioselective Michael addition of an N-protected aminomethylenephosphonate onto acrylic acid derivatives and trans-β-nitrostyrene. Among these crown ethers, three are new. Michael adducts were formed with good to excellent enantio- and diastereoselectivities. Combined MM and QM calculations have revealed that suitable side arms on the crown ether beneficially affect the position of the central sodium cation, which in turn helps enhance the stereocontrol by allowing a closer contact between substrate and catalyst.


 Please wait while we load your content...
Please wait while we load your content...