Three novel mononuclear Mn(iii)-based magnetic materials with square pyramidal versus octahedral geometries†
Abstract
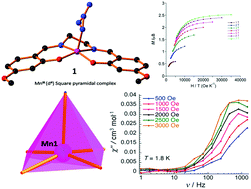
A series of new manganese schiff base complexes have been prepared and characterized by single crystal X-ray diffraction studies, which showed that all the three complexes are mononuclear; 1 and 2 have square pyramidal geometry, whereas 3 has an octahedral geometry. Magnetic studies reveal that all these complexes exhibit (i) moderate frequency dependence of ac magnetic susceptibility at low temperature upon the application of a dc external field; (ii) significant magnetic anisotropy (D) and (iii) antiferromagnetic interaction that persist between the neighbouring molecules. Therefore, our systematic studies have shown the influence of electronic, geometric and packing effects of a new series of mononuclear manganese(III) complexes with axially elongated square pyramidal and octahedral geometries on the negative D values.


 Please wait while we load your content...
Please wait while we load your content...