A spectroscopic and molecular dynamics simulation approach towards the stabilizing effect of ammonium-based ionic liquids on bovine serum albumin†
Abstract
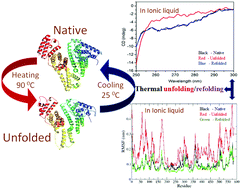
In this article, we have explored the impact of ammonium-based ionic liquids (ILs) on the thermal unfolding/refolding of bovine serum albumin (BSA) in aqueous solutions using thermal circular dichroism (CD) spectroscopy and molecular dynamic simulation studies. In attempting to find ILs that can stabilize BSA at higher temperatures, we found good results with ammonium-based ILs. Our results show that the hydrophobicity of the IL is very crucial in the refolding phenomenon. A more hydrophobic IL, triethylhexylammonium bromide, shows better refolding of thermally denatured BSA and the stabilization is found to be dependent on the concentration of the IL. Moreover, fluorescence measurements (synchronous, life time, 8-anilino-1-naphthalenesulfonic acid (ANS)) were used to decipher the conformational changes of the protein in the IL medium. The spectroscopic studies suggest that the native state of BSA is not altered in the IL medium, rather a compact structure of BSA is established which is further supported by the molecular dynamics simulation analysis. In addition, the esterase-like activity of BSA was studied in the IL medium and the possible binding sites were investigated using a molecular docking program. We hope that the present study is successful in interpreting the possible mechanism of interaction between BSA and ILs as well as the stabilizing/destabilizing effect of ILs on BSA.


 Please wait while we load your content...
Please wait while we load your content...