Synthesis of diastereomeric anhydrides of (RS)-ketorolac and (RS)-etodolac, semi-preparative HPLC enantioseparation, establishment of molecular asymmetry and recovery of pure enantiomers†
Abstract
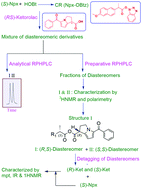
Herein, enantioseparation of two anti-inflammatory drugs, namely, (RS)-ketorolac and (RS)-etodolac, commonly marketed and administered as racemates, was achieved by RP-HPLC. This method provided very low limit of detection values (3.69 and 3.02 ng mL−1 for diastereomeric derivatives of (R)- and (S)-Ket, respectively) as compared to those reported in literature. (S)-Naproxen benzotriazole ester, which was used as a chiral reagent, was synthesized and characterized by UV, IR, and 1H NMR spectroscopies, elemental analysis, and polarimetry. The diastereomeric derivatives were synthesized via microwave irradiation, separated on an analytical scale by RP-HPLC, and then isolated by preparative HPLC. The use of a mobile phase containing methanol and aqueous triethylamine phosphate (TEAP) in the isocratic mode was found to be successful for the separation of diastereomeric derivatives, and the separation conditions with respect to pH, flow rate, and buffer concentration were optimized. The diastereomeric derivatives were characterized, and their absolute configuration was established. Hydrolysis of the derivatives provided native enantiomers under mild reaction conditions. This study describes the successful enantioseparation of the above mentioned two analytes by semi-preparative HPLC with easy recovery of the native enantiomers without racemization and with the establishment of molecular asymmetry.


 Please wait while we load your content...
Please wait while we load your content...