Noncovalently bound complexes of polar molecules: dipole-inside-of-dipole vs. dipole–dipole systems†
Abstract
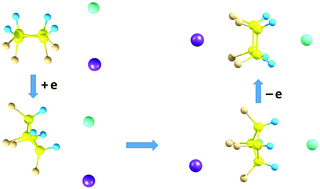
A series of molecular complexes including the uncommon M-mol-X species with one molecule (fluorocarbon) trapped between fragment counter-ions of the other (alkali halide MX), are compared to the usual mol-MX conformers with molecules attached to one another. The systems are characterized in terms of structures and stabilities, charge distributions and dipole moments, IR intensity spectra and electron attachment. Significant variations in the relative stabilities of the conformers are found between the systems with trapped-molecule isomers of different polarity as well as between neutral systems and their anions. In particular, it is shown that the M-mol-X conformers can be degenerate or perhaps even more stable relative to the mol-MX ones, as well as that the higher-energy isomer of the molecule can form a more stable complex. The electron-attachment is predicted to favour production of such uncommon species experimentally via making them very close in energy to the usual complexes and lowering the relevant energy barriers. The systems are highly polar and their IR intensity spectra are demonstrated not only to sensitively indicate the complex formation, but also to distinguish the specific conformers. The predicted features are hoped to aid experimental detection of novel species.


 Please wait while we load your content...
Please wait while we load your content...