A convenient and label-free colorimetric assay for dopamine detection based on the inhibition of the Cu(ii)-catalyzed oxidation of a 3,3′,5,5′-tetramethylbenzidine–H2O2 system
Abstract
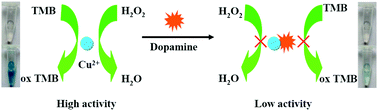
In this study, a simple and label-free colorimetric sensing strategy was reported for rapid and selective detection of dopamine by inhibiting the Cu2+-catalyzed oxidation of a 3,3′,5,5′-tetramethylbenzidine (TMB)–H2O2 system. Similar to natural peroxidase, Cu2+ could catalyze the oxidation of the peroxidase substrate TMB to oxidized TMB (ox TMB) in the presence of H2O2, producing a blue color. However, dopamine could chelate with Cu2+ to form stable dopamine–Cu2+ complexes by strong coordination between Cu2+ and dopamine. It was found that the formation of dopamine–Cu2+ complexes hindered the Cu2+-catalyzed oxidation of the TMB–H2O2 system. As a result, in the presence of dopamine, the solution color changed from blue to colorless with a remarkable decrease of absorbance. Under optimized experimental conditions, the colorimetric sensor exhibited a linear range of 1 μM to 50 μM for dopamine detection, with a detection limit of 1 μM. Furthermore, the proposed method was successfully applied to determine the dopamine content in real samples. Compared with other reported methods, the colorimetric method could be performed within several minutes and did not require any complex or time-consuming preparation and purification process. The results suggested that this strategy would have potential applications in biotechnology and clinical diagnosis.


 Please wait while we load your content...
Please wait while we load your content...