Bis(4-nitroanilines) in interactions through a π-conjugated bridge: conformational effects and potential molecular switches†
Abstract
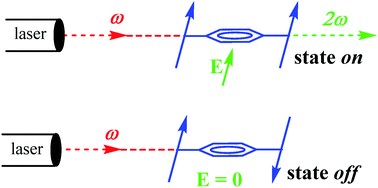
Two molecules, 2,2′-(1,4-phenylenebis(ethyne-2,1-diyl))bis(4-nitroaniline) (PNA) and 2,2′-(1,4-phenylene-bis(ethyne-2,1-diyl))bis(N,N-diethyl-4-nitroaniline) (PDNA), built up from two p-nitroaniline “push–pull” nonlinear optical (NLO) chromophores slightly conjugated through a 1,4-phenylenebis(ethyne-2,1-diyl) bridge have been synthesized and fully characterized. The crystal structure of PNA reveals that the ground (off) state conformation corresponds to the centrosymmetric anti-conformer in which the molecular hyperpolarizability (β) is equal to zero. A computational investigation reveals that, once submitted to an intense electric field (E), the two p-nitroaniline subunits are gradually aligned in the direction of the field with the appearance of a metastable (on) state (β ≠ 0, when E = 0), stabilized by conjugation. A loop of hysteresis is observed during the on ![[leftrightharpoons]](https://www.rsc.org/images/entities/char_21cb.gif) off cycles leading to a form of molecular bistability. The NLO response of PNA has been investigated at different field intensities by the electric-field induced second-harmonic (EFISH) technique to provide experimental evidence for this behavior.
off cycles leading to a form of molecular bistability. The NLO response of PNA has been investigated at different field intensities by the electric-field induced second-harmonic (EFISH) technique to provide experimental evidence for this behavior.


 Please wait while we load your content...
Please wait while we load your content...