Nitrogen-doped star-shaped polycyclic aromatic hydrocarbons based on fused triazatruxenes: synthesis and optoelectronic properties†
Abstract
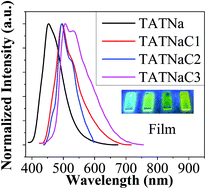
A new class of nitrogen-containing polycyclic aromatic hydrocarbons (N-PAHs) based on naphthalene-fused triazatruxenes, named as TATNaCn (n = 1–3), were successfully designed, synthesized and characterized. During the oxidative cyclodehydrogenation, multiple dehydrocyclization molecules can be effectively separated in a single reaction, implying a stepwise ring-closing process. A combination of thermal, optical, and electrochemical characterization of these stepwise ring-closed N-PAHs was conducted to explore the influence of the ring-fusing process on the optoelectronic characteristics of N-PAHs. The absorption bands of the fused products, TATNaCn, are red-shifted compared to those of the TATNa precursor, both in dilute solution and thin solid films, suggesting enlarged π-conjugation induced by the fused rings. Furthermore, the band-gap energies of these N-PAHs are facilely modulated by varying the number of closed rings, leading to tunable emission characteristics. This method of introducing triazatruxenes to construct N-PAHs provides general guidelines for exploring novel heteroatom-doped PAHs with facilely tunable optoelectronic properties.


 Please wait while we load your content...
Please wait while we load your content...