Ni@M (M = Pt, Pd and Ru) core@shell nanoparticles on a Vulcan XC-72R support with superior catalytic activity toward borohydride oxidation: electrochemical and fuel cell studies
Abstract
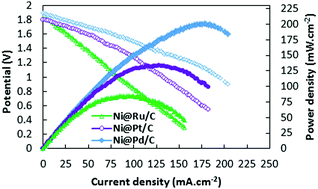
Ni@Pt, Ni@Pd and Ni@Ru nanoparticles with a core@shell structure were synthesized using a two-step reduction method on a Vulcan XC-72R support using sodium borohydride (NaBH4) and ethylene glycol (EG) as reducing agents for each step, respectively. The metal loading in electrocatalysts was 20 wt% and the molar ratio of Ni to M (M = Pt, Pd and Ru) was 1 : 1. The morphology of the synthesized electrocatalysts was characterized using field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) and fast Fourier transformation (FFT) methods. The electrocatalytic activity of the synthesized electrocatalysts toward borohydride oxidation in alkaline medium was investigated using cyclic voltammetry (CV), chronopotentiometry (CP) chronoamperometry (CA) and electrochemical impedance spectroscopy (EIS). The results showed that Ni@Pd/C has the highest electrochemically active surface area (ECSA) (164.9 m2 g−1) that is 1.69 and 2.39 times higher than Ni@Pt/C (97.3 m2 g−1) and Ni@Ru/C (69.1 m2 g−1), respectively. Also, Ni@Pd/C showed superior catalytic activity toward borohydride oxidation (29 022 A g−1) that is 1.32 and 8.55 times higher than Ni@Pt/C (22 016 A g−1) and Ni@Ru/C (3394 A g−1) catalysts, respectively. A Membrane Electrode Assembly (MEA) was fabricated with the catalyst-coated membrane (CCM) technique using Ni@Pt/C, Ni@Pd/C and Ni@Ru/C as anodic electrocatalysts and Pt/C as a cathodic electrocatalyst. The results showed that the fuel cell with the Ni@Pd/C electrocatalyst has the maximum power density (200.78 mW cm−2).


 Please wait while we load your content...
Please wait while we load your content...