Low-temperature CO oxidation over CeO2 and CeO2@Co3O4 core–shell microspheres
Abstract
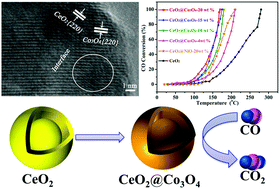
Mesoporous CeO2 core–shell microspheres were obtained by pyrolysis of a Ce–asparagine coordination polymer precursor. Then, upon a sheath-coating process via solvothermal treatment with Co(CH3COO)2·4H2O, Co3O4 species were uniformly dispersed on the CeO2 core–shell microsphere surface to generate the CeO2@Co3O4 composite. When used as catalysts for the oxidation of CO to CO2, the temperature required for full CO conversion was 280 °C and 170 °C for the core–shell microspheres CeO2 and CeO2@Co3O4-20 wt%, respectively. The porous structure with a core–shell architecture provided a larger surface area and abundant oxygen vacancies to adsorb and activate the CO molecules, resulting in an enhanced catalytic activity. Moreover, the CeO2@Co3O4 core–shell microspheres could maintain complete CO conversion after 30 h reaction. The enhanced CO conversion performance of the CeO2@Co3O4 core–shell microspheres was ascribed to the synergistic interaction between Co3O4 and CeO2.


 Please wait while we load your content...
Please wait while we load your content...