The ground and excited-state electronic structures of sandwich compounds Cp2(ME)2 contain an (ME)2 four-membered ring (Cp = C5H5; M = Ni, Pd, Pt; E = O, S, Se, Te)†
Abstract
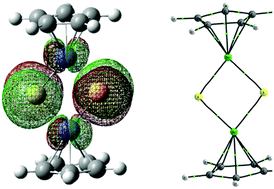
The structure, nature of chemical bonds and aromatic properties of Cp2(ME)2 (M = Ni, Pd, Pt; E = O, S, Se, Te) compounds have been studied using density functional theory. The charge transfer from the ground state (GS) to the vertically excited state (VES) as well as the differences in the structure and nature between the GS and the adiabatically excited state (AES) are discussed in this work. The results show that the HOMO (highest occupied molecular orbital)–LUMO (lowest unoccupied molecular orbital) gaps of the studied compounds are small. Hence, the electron in the HOMO is easily excited. The electron transition from the HOMO to the LUMO, the strength and nature of the M–E bond along with the M–Cp and E⋯E interactions and the aromaticity of the (ME)2 (M = Ni, Pd, Pt; E = O, S, Se, Te) rings are correlated with the periods of the M and E atoms. In addition, electronic excitation enhances the chemical bond strength in the (ME)2 ring.


 Please wait while we load your content...
Please wait while we load your content...