A terbium(iii)-based coordination polymer for selective and sensitive sensing of nitroaromatics and ferric ion: synthesis, crystal structure and photoluminescence properties†
Abstract
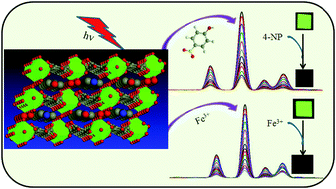
A potent luminescent sensor for the detection of 4-nitrophenol (4-NP) and Fe3+ ion was developed based on a terbium(III)-based coordination polymer, [Tb(H2O)5(BTEC)][H(C5H6N2)]·3H2O (1), which was prepared under hydrothermal condition using a benzene-1,2,4,5-tetracarboxylate (BTEC) bridging ligand. 1 possesses one-dimensional zig-zag chains decorated with carboxylate functional sites and protonated 4-aminopyridine guests located in interlayer channels. The strong green emission of 1 could be efficiently quenched by trace amounts of 4-NP through a luminescence quenching mechanism even in the presence of other aromatic compounds such as 1,2-dimethylbenzene, bromobenzene, phenol, 4-bromophenol, 4-nitrotoluene, 1,2-dinitrobenzene, nitrobenzene, 2,6-dinitrotoluene, and 2,4,6-trinitrophenol. Furthermore, 1 exhibits superior luminescence quenching behavior towards Fe3+ ion over other competing interfering metallic ions such as Cr3+, Cu2+, Na+, Mg2+, Mn2+, Co2+, Fe2+, Pb2+, Pd2+, and Zn2+. The possible luminescence quenching mechanisms were also discussed. Moreover, visual fluorescent test papers based on 1 were fabricated and could be utilized for selective detection of nitroaromatic compounds and metal ions.


 Please wait while we load your content...
Please wait while we load your content...