Self-assembly of polyoxometalate-based hybrid aggregates: from a monomer to dimers by changing the pH value of reaction systems†
Abstract
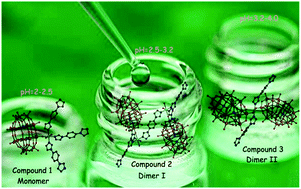
Three new compounds have been synthesized at different pHs under identical hydrothermal conditions, namely, (H2bimb)[Cu(bimb)3(H2O)2(H2P2W18O62)]·4H2O (1), (H2bimb)3[Cu2(bimb)5(H2O)4(HP2W18O62)2]·9H2O (2), and (H2bimb)5[Cu(bimb)2(H2O)2(P2W18O62)2]·8H2O (3) (bimb = 4-bis(imidazol-1-yl)benzene). Their structures were determined using single crystal X-ray diffraction analyses and characterized using routine methods. When the pH was adjusted to 2.0–2.5, compound 1 was obtained, which is a monomer. Compounds 2 and 3 were obtained at pH values of 2.5–3.2 and 3.2–4.0, which are two kinds of distinct dimers (I and II), respectively. The pH of the reaction plays a key role in the assembly of POM-based hybrid aggregates. Electrochemical experiments indicate that a 1-based carbon paste electrode (1-CPE) exhibits high catalytic efficiency and high stability towards reduction of NO2−. Namely, the electrocatalytic efficiency towards the reduction of NO2− is ca. 235% in 1 M H2SO4 containing 50 mM NO2−, and the current signal after 100 cycles exhibits almost no loss for the 1-CPE.


 Please wait while we load your content...
Please wait while we load your content...