Palladacycles having normal and spiro chelate rings designed from bi- and tridentate ligands with an indole core: structure, synthesis and applications as catalysts†
Abstract
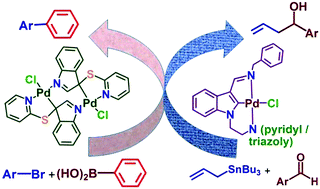
1-Pyridin-2-ylmethyl-1H-indole-3-carbaldehyde and 1-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1H-indole-3-carbaldehyde were synthesized. Their condensation with benzyl amine resulted in indole core containing Schiff bases benzyl-(1-pyridin-2-ylmethyl-1H-indol-3-ylmethylene)amine (L1) and benzyl-[1-(1-benzyl-1H-[1,2,3]triazole-4-ylmethyl)-1H-indol-3-yl methylene]amine (L2) respectively, unknown hitherto. The K2CO3-promoted sulfenylation of the indole formed 3-(pyridin-2-ylsulfanyl)-1H-indole (L3), also unknown so far. The yield of L1–L3 was 72–93%. L1 and L2 on reaction with sodium tetrachloropalladate(II) in the presence of CH3COONa give complexes [Pd(L1/L2-H)Cl] (1/2) in which they bind in a tridentate (N, C−, N′) pincer mode. L3 on reaction with [(MeCN)2PdCl2] results in a dimeric palladacycle [Pd(L3-H)Cl]2 (3) with a spiro ring. The precursor aldehydes, L1–L3 and the Pd(II)-complexes derived from them, were characterized using 1H and 13C{1H} NMR and HR-MS. Complexes 2 and 3, ligands L1 and L3 and the precursor aldehydes of L1 and L2 were authenticated with single crystal X-ray diffraction. The Pd–C bond distances (Å) are 1.932(8)/2.115(3) (2/3). The Pd–N bond lengths (Å) are: 2.063(7) and 2.028(7) for 2 and 2.053(3) and 2.019(3) for 3. These complexes have been found to be efficient as catalysts for the Suzuki–Miyaura coupling of ArCl (3 as a catalyst) and ArBr and allylation of a variety of aldehydes (1 and 2 as catalysts). The optimum loading of the complexes as catalysts is 0.001–0.01 and 1 mol% respectively for the two reactions, which appear to follow a homogeneous pathway.


 Please wait while we load your content...
Please wait while we load your content...