Fluorescent phenanthroimidazoles functionalized with heterocyclic spacers: synthesis, optical chemosensory ability and two-photon absorption (TPA) properties†
Abstract
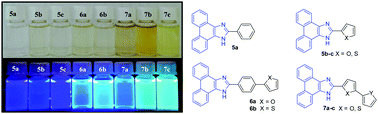
Three series of fluorescent phenanthroimidazoles bearing heterocyclic spacers were synthesized in moderate to excellent yields and evaluated as optical chemosensors for ions as well as two-photon absorbing chromophores. Interaction of compounds 5–7 with anions and cations in acetonitrile and acetonitrile/H2O (95 : 5) showed them to be selective receptors for several anions (AcO−, CN− and F−) and cations (Fe3+, Cu2+ and Pd2+), with compound 7a being the most sensitive receptor for Fe3+ and Cu2+. On the other hand, compounds 5a, 7b and 7c were the most sensitive receptors for AcO−, CN− and F−. The binding stoichiometry between the receptors and the anions and cations was found to be 1 : 2 (ligand to anion/metal cation). The binding process was also followed by 1H NMR titrations. The evaluation of the TPA properties of chosen phenanthroimidazoles 7a–c by the two-photon induced fluorescence method revealed that compound 7c, which contains a bithienyl spacer, exhibited the highest TPA cross-section (σ2) value.


 Please wait while we load your content...
Please wait while we load your content...