Exploring the gem-dimethyl effect in the formation of imine-based macrocycles and cages†
Abstract
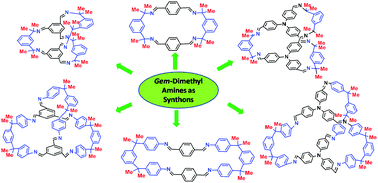
Using the gem-dimethyl effect as a platform, a variety of organic [2+2] macrocycles and [2+3] cofacial cages was obtained in high yields. Imine-based macrocyclization could be performed even at high concentrations (0.5 to 1.0 M), via instant mixing of aldehydes and amines.


 Please wait while we load your content...
Please wait while we load your content...