Cu-Catalyzed first direct access towards 3-sulfenylindoles from aryl halides†
Abstract
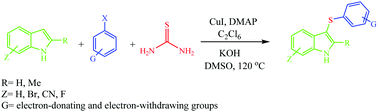
Copper iodide has been used as a catalyst for developing a new route to 3-sulfenylindoles from the reaction of aryl halides with indole derivatives in the presence of thiourea and hexachloroethane in DMSO at 120 °C in less than an hour.


 Please wait while we load your content...
Please wait while we load your content...