CsF-promoted carboxylation of aryl(hetaryl) terminal alkynes with atmospheric CO2 at room temperature†
Abstract
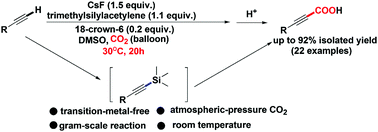
A CsF-promoted carboxylation of aryl(hetaryl) terminal alkynes with atmospheric CO2 in the presence of trimethylsilylacetylene was developed to give functionalized propiolic acid products at room temperature. A wide range of propiolic acids bearing functional groups was successfully obtained in good to excellent yields. Mechanistic studies demonstrate that in the carboxylation process the alkynylsilane intermediate was first in situ generated, which was then trapped by CO2, giving rise to the corresponding functionalized propiolic acids after acidification. The advantages of this approach include avoiding use of transition-metal catalysts, wide substrate scope together with excellent functional group tolerance, ambient conditions and a facile work-up procedure.


 Please wait while we load your content...
Please wait while we load your content...