A coordinatively saturated nickel complex supported by triazenido ligands: a new electrocatalyst for hydrogen generation via ligand-centered proton-transfer†
Abstract
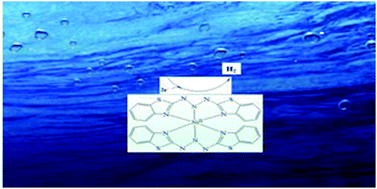
It is regarded that transition metal complexes can catalyze hydrogen production via an unstable metal-hydride species, and the reduction reaction occurs at the metal center. In this paper, we present a new catalytic mechanism via ligand-centered proton-transfer for hydrogen generation catalyzed by a coordinatively saturated nickel complex, [NiII(L)2] 1, which is obtained by the reaction of the triazenido ligand, 1,3-bis[2-aminobenzothiazole]triazene (HL), with Ni(CH3CO2)2. Electrochemical investigations show that the electrocatalytic system based on [NiII(L)2] can afford 76.31 and 685.76 moles of hydrogen per mole of catalyst per hour (mol H2/mol catalyst/h) from acetic acid at an overpotential (OP) of 991.6 mV and from a neutral aqueous buffer (pH 7.0) at an OP of 837.6 mV, respectively.


 Please wait while we load your content...
Please wait while we load your content...