Temperature-dependent water penetration in Tween20:cholesterol niosome membrane: a study using excited state prototropism of 1-naphthol†
Abstract
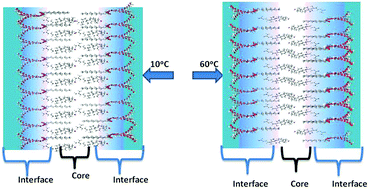
This work uses the dynamics of the excited state proton transfer of 1-naphthol to water as a basis for understanding the temperature-dependent changes in the water accessibility of the niosome membrane domain. The decrease in the neutral form fluorescence intensity of membrane bound 1-naphthol, the significant drop in the membrane-bound anion form fluorescence lifetime, and the changes in the fluorescence lifetime distribution plots of the two prototropic forms of 1-naphthol indicate a regular increase in the water penetration in the interfacial region of the niosome membrane domain, whereas the dry core regions appear unaffected with increasing temperature (10–60 °C). Unlike some liposomes and niosomes, these niosomes do not show thermotropic phase transition behavior.


 Please wait while we load your content...
Please wait while we load your content...