Pd-PEPPSI-IPentCl: a new highly efficient ligand-free and recyclable catalyst system for the synthesis of 2-substituted indoles via domino copper-free Sonogashira coupling/cyclization†
Abstract
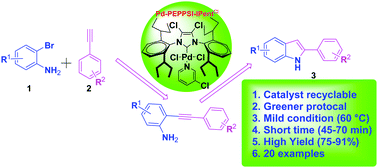
A pyridine-containing decidedly resourceful Pd-N-heterocyclic carbene complex, Pd-PEPPSI-IPentCl (PEPPSI = pyridine enhanced precatalyst preparation, stabilization, and initiation), is used as a first class recyclable catalytic system for the synthesis of 2-substituted indoles via domino copper-free Sonogashira coupling/cyclization. The catalyst showed a greater performance in the cascade reaction of various 2-bromo anilines with different terminal acetylenes under mild (60 °C) and green conditions (ethanol : water) even in the absence of a copper catalyst and an inert atmosphere. The catalyst is used for the first time in these reactions. The findings suggest that 0.1 mol% of the catalyst is sufficient, and that the catalyst is recyclable and can be reused for up to six cycles.


 Please wait while we load your content...
Please wait while we load your content...