Experimental and DFT studies of disubstituted 2-(2-hydroxyphenyl)benzothiazole-based fluorophores synthesized by Suzuki coupling†
Abstract
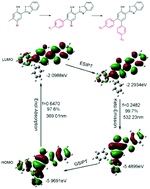
Among the various fascinating fluorescent compounds, excited-state intramolecular proton transfer (ESIPT) dyes have been drawing much attention due to their anomalously large Stokes shifts. Furthermore, ESIPT fluorophores are attractive as their fluorescence properties may be fine-tuned through facile chemical modifications of existing dyes. Varying the strength of the electron-donating and electron-withdrawing substituents anchored at different sites of the parent molecules, especially when incorporating these substituents at several different sites simultaneously, is an effective way to achieve targeted fluorescence properties. In the present study, a new family of 2-(2-hydroxyphenyl)benzothiazole (HBT)-based disubstituted derivatives, with substitutions at the 4′- and 5′-positions, were readily synthesized by the Suzuki coupling reaction. It was found that the electronic effects of the 5′-position substituents on the molecular orbital levels were opposite to that of the 4′-position substituents. Theoretical calculations demonstrate that the substituents at the 4′- and 5′-positions dramatically affect the HOMOs and the LUMOs, respectively, which is in conformity with experimental results and can be applied to predict shifts in fluorescence emission. Additionally, these derivatives show highly efficient keto-emission in the aggregated state. The X-ray crystallographic analysis shows that weak intramolecular interactions, which restrict intramolecular rotation, and strong intramolecular hydrogen bonding, which facilitates intramolecular proton transfer, are responsible for the intense keto-emission observed. Our study may provide experimentally and theoretically valuable instructions for designing other high-performance ESIPT fluorophores to meet the demands of specific applications.


 Please wait while we load your content...
Please wait while we load your content...